Antiviral Tecovirimat Shows Limited Efficacy in Recent Clinical Trials
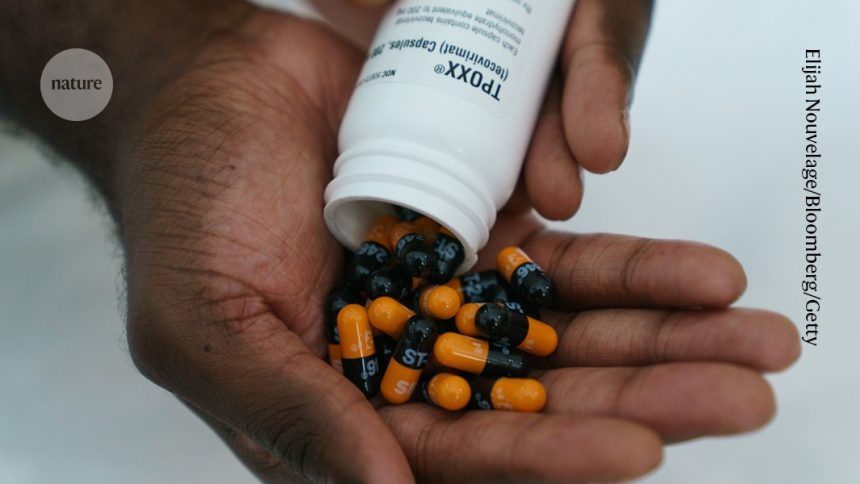
Published in Nature on August 16, 2024, a new study reveals that the antiviral medication tecovirimat may not offer significant benefits compared to a placebo when combating the clade I virus variant. The findings indicate challenges in effectively treating infections caused by this particular strain.
Study Insights and Background
This clinical trial aimed to determine the efficacy of tecovirimat, an antiviral drug previously believed to hold promise against various viral infections. However, early outcomes suggest that it does not significantly outperform placebo treatments for patients infected with clade I variants. This news is particularly noteworthy given the ongoing global concern regarding viral outbreaks and their management.
The Importance of Clade Variants
The study highlights an essential aspect of virology: the emergence of different virus variants can complicate treatment protocols. In recent years, several viral strains have emerged, raising questions about existing antiviral agents’ effectiveness. Clade I is just one of many types being monitored by researchers as they strive to develop more effective therapeutic strategies.
Current Implications for Antiviral Research
The inadequate response observed with tecovirimat against clade I points toward a critical need for further research into alternative treatments or novel anti-viral therapies tailored specifically for resistant strains. As per health experts in epidemiology, focusing on understanding these evolving virus types could lead to breakthroughs necessary for effective management.
Navigating Future Research Opportunities
This outcome presents an opportunity for scientists and pharmaceutical companies alike to explore innovative pathways and potentially uncover new antiviral mechanisms tailored explicitly toward diverse clades and their unique attributes. Emerging technologies like machine learning could play a crucial role in predicting virus mutations and crafting focused therapies moving forward.